
Difference Between Solutions and Suspensions, General Science Lecture
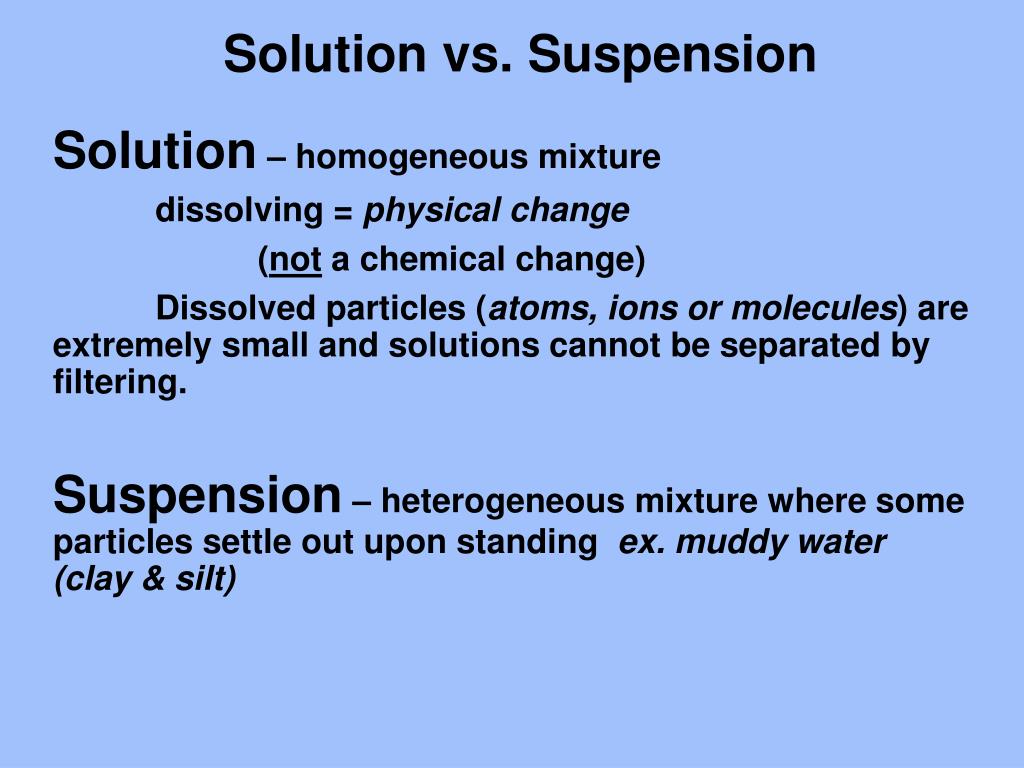
Suspension vs Solution The solution is a homogeneous mixture of two or more substances, molecules, ions, or atoms dissolved in a solvent. A suspension is a heterogeneous mixture of two or more substances dispersed throughout a liquid or gas, with larger particles that are not fully dissolved.
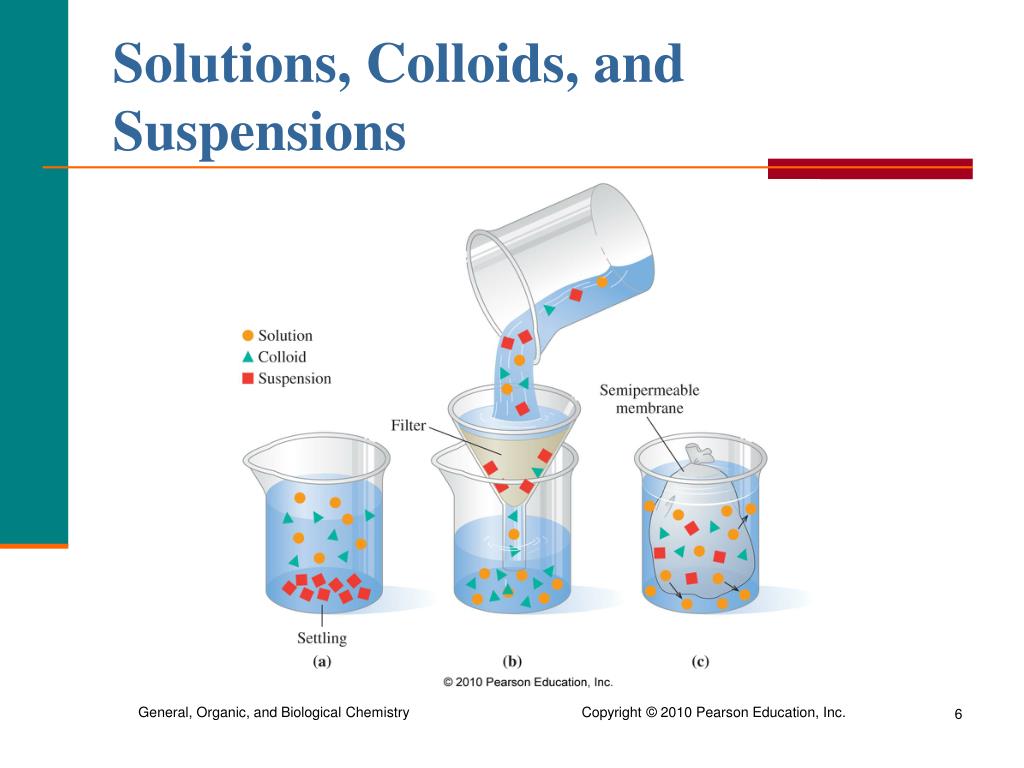
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID
Suspensions - A mixture that contains solid particles large for sedimentation. Emulsions - A mixture of two or more liquids that are unmixable. FACT: Water is considered a universal solvent. It has the ability to dissolve more substances than any other solvent.

abri Réfléchi Labyrinthe solution suspension colloid mental Tout faire
1.Solutions are mixtures that are homogeneous while suspensions are mixtures that are heterogeneous. 2.The particles of a solution are at the ion or molecular level and cannot be seen by the naked eye while the particles of a suspension can be seen by the naked eye.

PPT Solution vs. Suspension Demonstration PowerPoint Presentation
A solution is a homogeneous mixture of two or more components. The dissolving agent is the solvent. The substance that is dissolved is the solute. The components of a solution are atoms, ions, or molecules, making them 10 -9 m or smaller in diameter. Example: Sugar and water Suspensions

PPT Properties of Matter PowerPoint Presentation, free download ID
Sol Solution vs. Suspension What's the Difference? Sol Solution and Suspension are both types of mixtures, but they differ in terms of their particle size and stability. Sol Solution is a homogeneous mixture where the solute particles are dispersed at the molecular or ionic level in a solvent.

PPT Solution vs. Suspension Demonstration PowerPoint Presentation
Most people are confused about what suspension solution is. Suspensions are obtained when insoluble solid particles are dispersed in a liquid medium. On shaking the solute particles can be evenly dispersed in the medium but if left undisturbed the solute particles which range from 0.5 to 5 μm tend to settle down and can be separated from the.
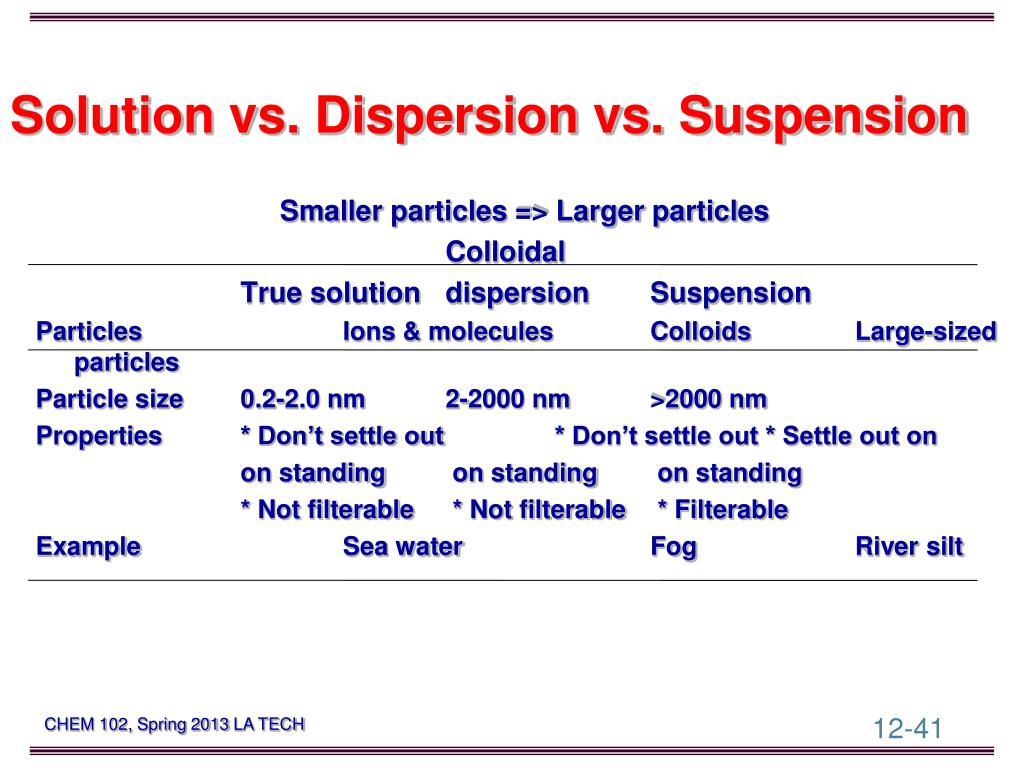
PPT Chemistry 102(01) Spring 2013 PowerPoint Presentation, free
Main Difference - Solution vs Suspension Solutions and suspensions are both considered as mixtures. The key difference between solution and suspension is their particle size. Particles in a solution are much smaller than that of suspensions.

What is the difference between a solution and a suspension? How are
Suspension refers to a heterogeneous mixture in which solid particles or liquid droplets are dispersed in a liquid or gas. Unlike solutions, the particles in a suspension do not dissolve but remain suspended or floating. Examples of Suspensions: Muddy water (small soil particles suspended in water) Paint (pigments suspended in a liquid medium)

Difference Between True Solutions, Colloidal solution and Suspension
Video transcript. Loading. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.

How are sol, solution and suspension different from each other
Solution vs. Suspension: What's the Difference? A solution is a homogeneous mixture where substances are dissolved, while a suspension is a heterogeneous mixture where particles are suspended but not dissolved. Key Differences A solution is a homogeneous mixture where the solute is completely dissolved in the solvent, forming a uniform composition.
PPT What is a homogeneous mixture? PowerPoint Presentation, free
Answer 1: The difference between a solution and a suspension is in the particle sizes involved. A solution is a mixture of ions or molecules (very, very small). Solutions are transparent, meaning that you can see through them. A suspension has bigger particle sizes and so it may look cloudy or murky.
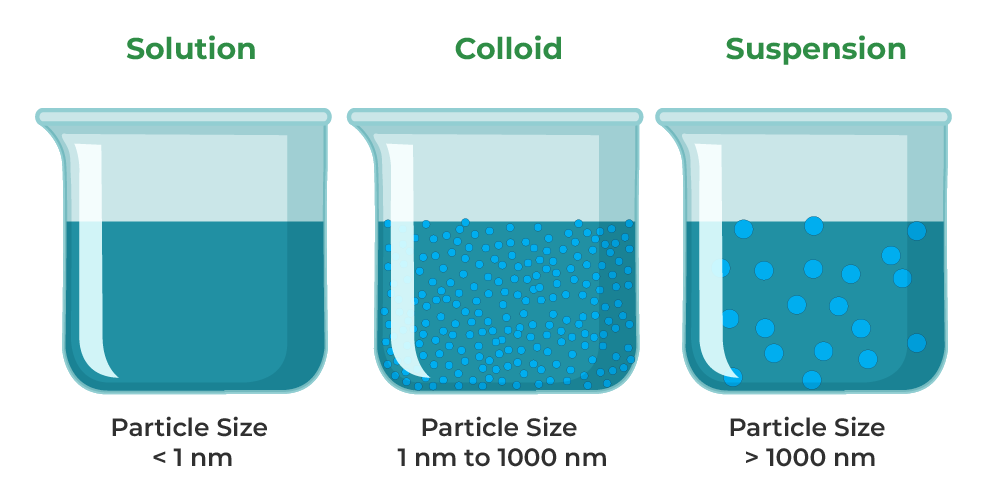
Suspension(Chemistry) Definition, Properties, Examples, and FAQs
Solution vs suspension As shown above, a solution consists of a solute dissolved in a solvent. This means that particles of the solute have been surrounded by solvent particles. This is a homogenous mixture. In contrast, a suspension is a mixture in which one component is not dissolved in the other. For example, sand and water.

Solutions Suspensions and Colloids Part 1/1 English Class 9 YouTube
Figure 7.6.1 7.6. 1: A mixture of sand and water forms a suspension. A suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon standing. The particles in a suspension are far larger than those of a solution, so gravity is able to pull them down out of the dispersion medium (water).
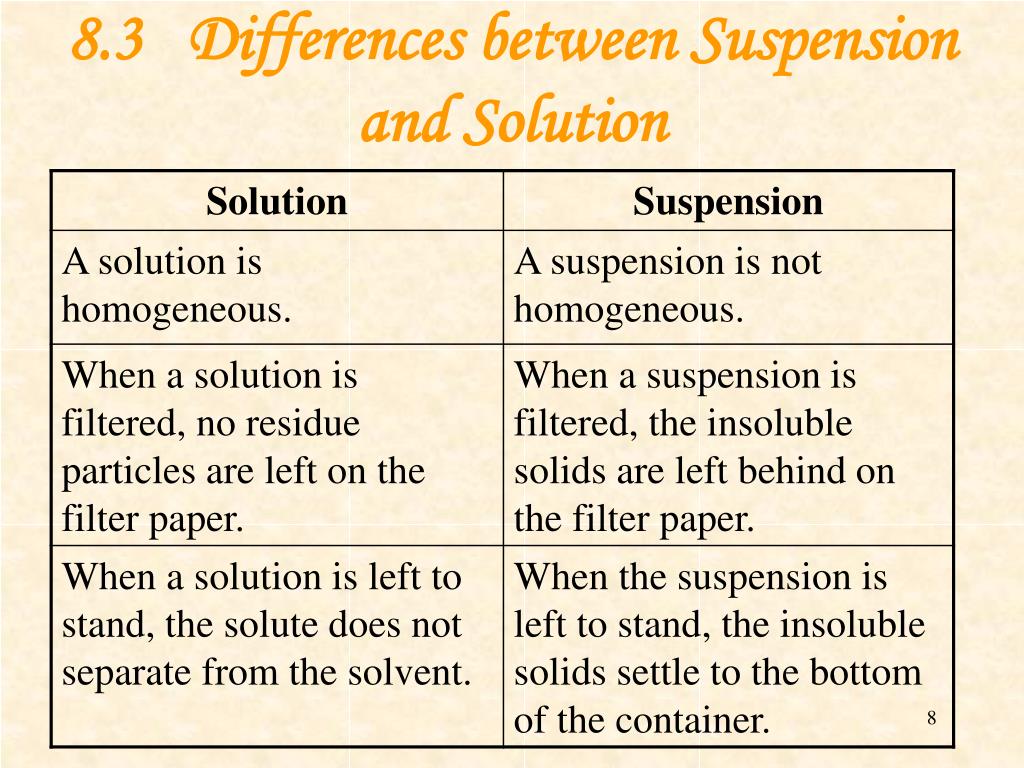
PPT Solution and Suspension PowerPoint Presentation, free download
Solution. These systems are homogenous, which means the solute and solvent have uniformity in them. In other words, they are evenly distributed in the entire system. Suspension. In contrast, suspensions are heterogeneous systems representing the non-uniformity of solute and solvent.

True Solution, Colloid solution and Suspension three different types of
Both solutions and suspensions are mixtures of two or more components and neither of them have components that are chemically bonded together. Components in both a solution and a suspension can be separated based on their physical properties of density, solubility or size. What Are Examples of Solutions?
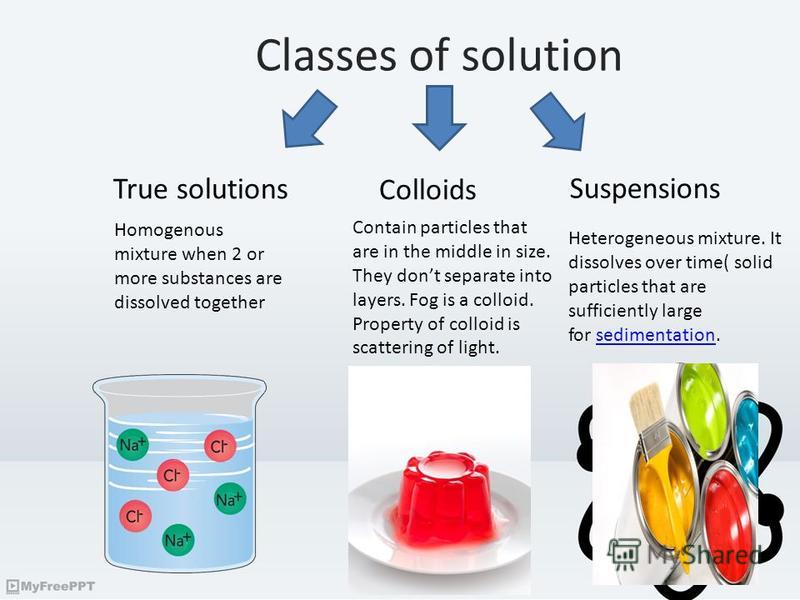
Презентация на тему "Colloidal systems. Classes of solution True
As nouns the difference between solution and suspension. is that solution is a homogeneous mixture, which may be liquid, gas or solid, formed by dissolving one or more substances while suspension is the act of suspending, or the state of being suspended.